Table of Contents
Introduction to Apoptotic pathway
- Apoptosis, also known as programmed cell death, is a fundamental process in multicellular organisms, playing a crucial role in development, tissue homeostasis, and immune response. Apoptotic pathway is a series of well-orchestrated signaling cascades that regulate the initiation and execution of apoptosis. Understanding these pathways is essential for comprehending cell death mechanisms and their implications in various diseases, including cancer and neurodegenerative disorders.
- Two primary apoptotic pathways exist: the intrinsic (mitochondrial) pathway and the extrinsic (death receptor) pathway. Both pathways converge on a common execution phase, leading to the dismantling of the cell and its eventual phagocytosis.
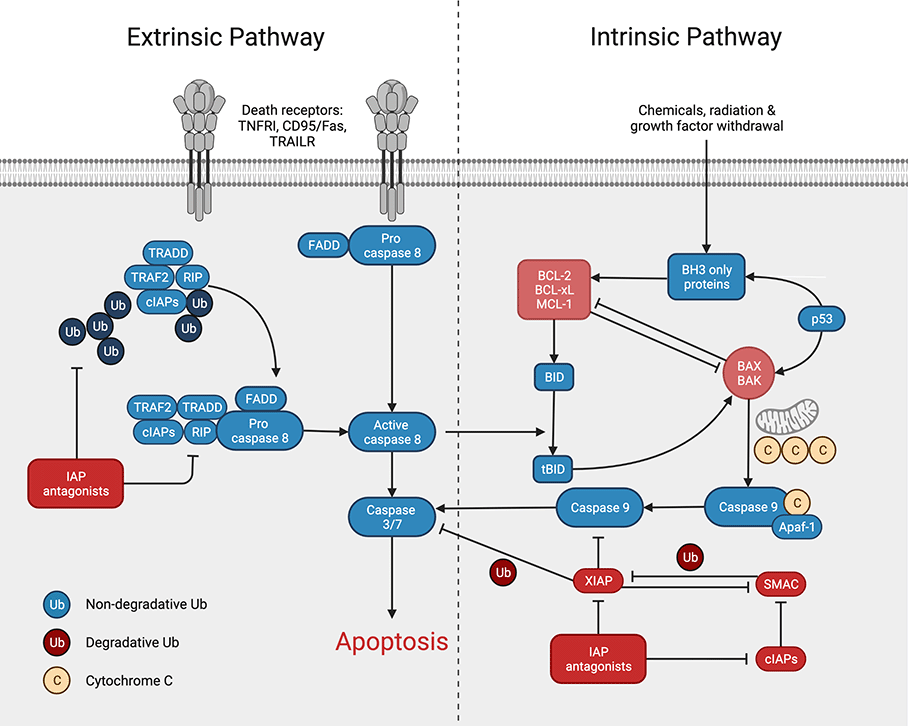
I. Extrinsic Pathway of Apoptosis
- The extrinsic pathway is triggered by signals from outside the cell, such as death receptors on the cell surface.
- These receptors, such as tumor necrosis factor (TNF) and Fas (CD95) receptors, bind to their respective ligands and undergo a conformational change that allows them to recruit adaptor proteins.
- These adaptor proteins, such as FADD and TRADD, recruit and activate the caspase cascade, which is a series of proteases that ultimately lead to the destruction of the cell.
- The initiator caspase in this pathway is caspase 8, which is activated by the adaptor proteins.
- Caspase 8 in turn activates the executioner caspases, caspase 3 and caspase 7, which cleave and degrade a variety of cellular proteins, leading to the characteristic morphological changes of apoptosis.
II. Intrinsic Pathway of Apoptosis
- The intrinsic pathway is activated by signals from within the cell, such as DNA damage or mitochondrial dysfunction.
- This pathway involves the release of pro-apoptotic molecules, such as cytochrome c and SMAC/DIABLO, from the mitochondria into the cytosol.
- These molecules bind to and activate the initiator caspase, caspase 9, which is a part of the Apaf-1 complex.
- Caspase 9 in turn activates the executioner caspases, caspase 3 and caspase 7, which cleave and degrade a variety of cellular proteins, leading to the characteristic morphological changes of apoptosis.
- The intrinsic pathway is also regulated by the Bcl-2 family of proteins, which can either promote or inhibit the release of pro-apoptotic molecules from the mitochondria.
- Both pathways ultimately activate caspases, proteases responsible for cleaving specific substrates that trigger cell destruction. Caspases cleave structural and regulatory proteins, leading to nuclear fragmentation, cytoplasmic blebbing, and DNA degradation.
- Apoptotic pathways are tightly regulated by pro-apoptotic and anti-apoptotic Bcl-2 family proteins, which govern the balance between survival and cell death. Dysregulation of these pathways can lead to abnormal cell death, contributing to various pathologies.
Conclusion
Studying apoptotic pathways provides insights into the molecular mechanisms of cell death and opens avenues for developing therapeutic interventions targeting apoptotic processes in diseases like cancer. Moreover, understanding these pathways can aid in the development of novel approaches for regenerative medicine and tissue engineering, where controlled apoptosis is critical for tissue remodeling and repair.