Table of Contents
Introduction:
Conformation of Protein refers to the three-dimensional structure of a protein. This determines its function, stability, and interactions with other molecules. Understanding this is essential for understanding the mechanisms of protein function and for the design of new drugs and therapies.
Discovery:
The study of protein conformation began in the early 20th century with the work of scientists such as Linus Pauling and J.D. Bernal. They used X-ray diffraction to determine the first protein structures, which laid the foundation for the field of protein structure determination.
Methods:
There are several methods used to determine the conformation of proteins, each with their own advantages and limitations. Some of the most common methods include:
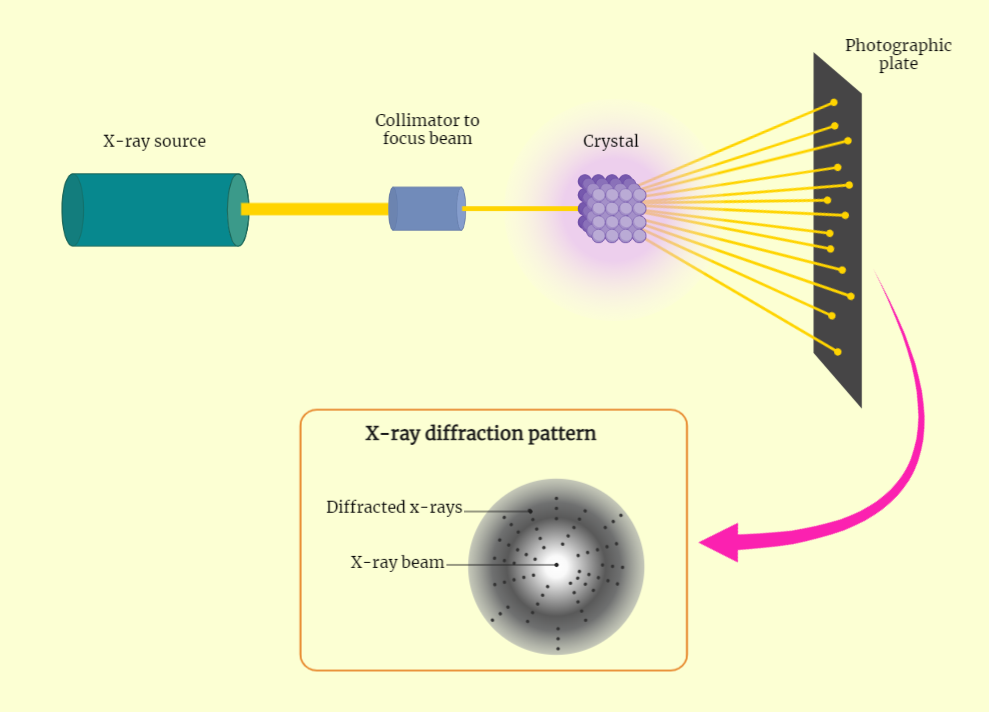
X-ray crystallography:
This method uses X-ray diffraction to determine the precise locations of atoms in a crystal of the protein. This method is highly precise, but requires large, well-ordered crystals of the protein.
Nuclear Magnetic Resonance (NMR) spectroscopy:
This method uses the magnetic properties of the nuclei of atoms to determine the structure of a protein in solution. This method does not require crystals, but is less precise than X-ray crystallography.
Cryo-electron microscopy (cryo-EM):
This method uses electrons to image a frozen hydrated protein sample, providing a 3D structure of the protein. It does not require crystals and can yield high resolution structures of large and flexible proteins.
Computational methods:
These methods use computer algorithms to predict the conformation of a protein based on its amino acid sequence. These methods can be used to predict the structure of an unknown protein in different environments or states.
Theory:
The conformation of a protein is determined by the interactions between its amino acid residues. These interactions include hydrogen bonds, electrostatic interactions, van der Waals interactions, and disulfide bridges. The stability and flexibility of a protein’s conformation are also influenced by the presence of non-covalent interactions such as hydrophobic interactions and electrostatic interactions.
Protein conformations can be classified into several types:
- The native conformation is the biologically active conformation of the protein.
- The denatured conformation is a conformation that the protein adopts when it is unfolded or denatured.
- Intermediate conformations are conformations that the protein adopts during the process of folding or unfolding.
Applications:
- Understanding Protein Function: Determining the conformation of a protein can provide insight into its function and mechanism of action.
- Drug Design: Understanding the conformation of a protein can aid in the design of new drugs and therapies by identifying potential binding sites or regions of structural instability that can be targeted by drugs.
- Protein Engineering: Understanding the conformation of a protein can be used to engineer new or improved proteins with specific functions or properties.
- Biotechnology: Determining the conformation of a protein can be used in the development of new biotechnology products such as enzymes, vaccines, and diagnostic tools.
In summary, the protein conformation is its three-dimensional structure. It is essential for understanding its function, stability and interactions with other molecules. There are several methods available to determine the conformation of proteins. Those are X-ray crystallography, NMR spectroscopy, cryo-EM and computational methods. Understanding the conformation of proteins has a wide range of applications in fields such as drug design, protein engineering and biotechnology.