Table of Contents
Introduction:
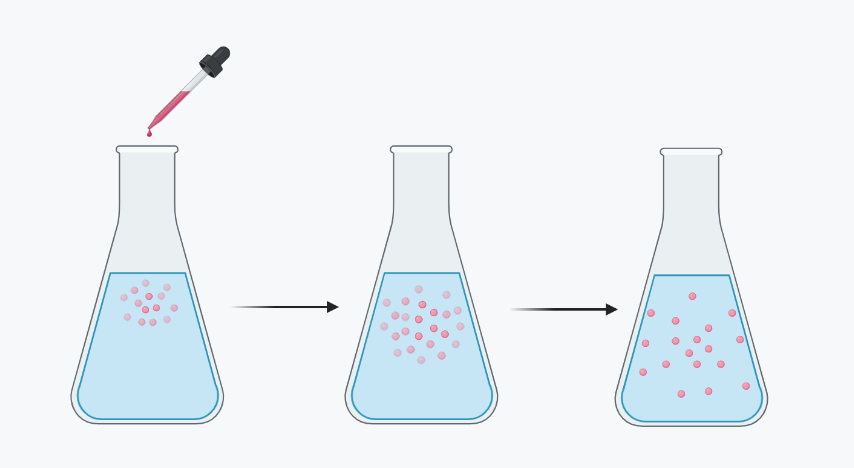
Diffusion is a fundamental process that plays a crucial role in various aspects of nature and life. It involves the spontaneous movement of particles or molecules from an area of high concentration to an area of low concentration, resulting in the equalization of concentration throughout the available space. It is driven by the random motion of particles and is a passive process that does not require external energy input.
Principles of Diffusion:
This process follows certain principles that govern its behavior:
- Concentration Gradient: Diffusion occurs due to the presence of a concentration gradient, which is the difference in the concentration of particles between two regions. The particles move from areas of higher concentration to areas of lower concentration until equilibrium is reached.
- Random Molecular Motion: The movement of particles in diffusion is random and results from their inherent kinetic energy. This random motion causes the particles to spread out and mix with surrounding particles.
- Net Movement: While individual particles move randomly, the overall effect of diffusion is a net movement of particles from higher to lower concentration. This movement continues until the concentration becomes uniform throughout the system.
Factors Affecting Diffusion:
Several factors influence the rate of this process:
- Temperature: Higher temperatures increase the kinetic energy of particles, leading to more rapid and energetic movement. As a result, diffusion occurs at a faster rate.
- Molecular Size: Smaller molecules diffuse more quickly than larger molecules because they have higher mobility and can pass through smaller gaps or pores in the medium.
- Medium: The nature of the medium through which diffusion occurs affects its rate. Diffusion is faster in mediums with lower viscosity and higher permeability, as they offer less resistance to the movement of particles.
- Concentration Gradient: A steeper concentration gradient leads to faster diffusion. The larger the difference in concentration between two regions, the more rapid the diffusion process.
Examples of Diffusion:
This process can be observed in various natural and everyday phenomena,
- Gas Exchange in the Lungs: During respiration, oxygen from inhaled air diffuses across the thin walls of the alveoli into the bloodstream, while carbon dioxide diffuses from the bloodstream into the alveoli to be exhaled.
- Osmosis in Plant Cells: Water diffuses through the cell membrane from an area of higher water concentration (hypotonic solution) to an area of lower water concentration (hypertonic solution) to maintain balance and regulate cell turgidity.
- Perfume Spreading: When perfume is sprayed in a room, its volatile molecules diffuse through the air, spreading and eventually reaching all corners of the room.
- Mixing of Solutions: When a drop of dye is added to a glass of water, the dye molecules disperse and diffuse throughout the water, resulting in the uniform coloring of the solution.
Conclusion:
Diffusion is a fundamental process that governs the movement of particles and molecules in various systems. It plays a significant role in biological processes, industrial applications, and everyday phenomena. Understanding the principles and factors influencing diffusion enhances our comprehension of how substances spread and equilibrate, contributing to a wide range of scientific disciplines.