Table of Contents
Definition:
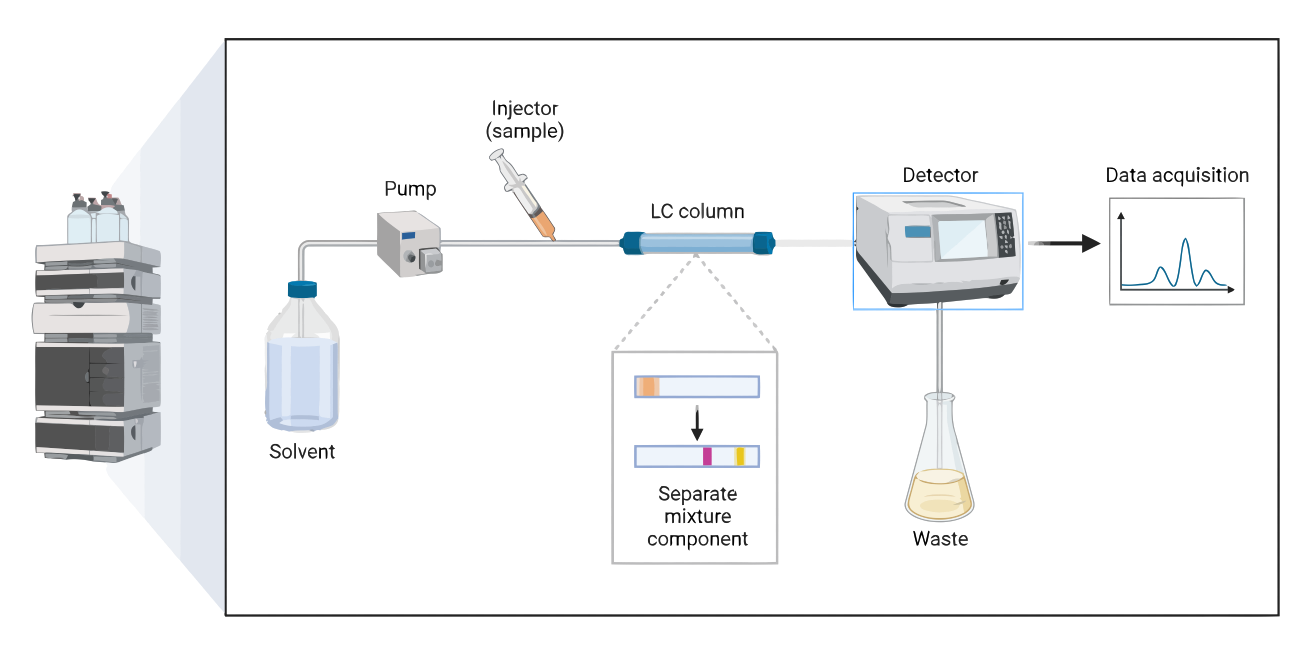
Liquid chromatography (LC) is a separation technique in which a mixture of compounds is passed through a stationary phase, typically a solid or a gel, and a mobile phase, which is a liquid. The compounds in the mixture interact differently with the stationary and mobile phases, leading to their separation.
Principle:
The principle of liquid chromatography is based on the differences in the distribution of the compounds between the stationary and mobile phases. The compounds in the mixture will distribute themselves between the two phases based on their relative affinities for each phase. The compounds with a higher affinity for the stationary phase will spend more time in contact with it and will be separated from those with a higher affinity for the mobile phase.
Types:
- Normal phase chromatography: In this type, the stationary phase is a polar material and the mobile phase is non-polar.
- Reverse phase chromatography: In this type, the stationary phase is a non-polar material and the mobile phase is polar.
- Ion exchange chromatography: In this type, the stationary phase is a solid matrix with ionizable groups and the mobile phase is a liquid containing ions.
Steps:
- Sample preparation: The sample is prepared by dissolving it in a suitable solvent.
- Sample injection: The prepared sample is injected into the chromatography system.
- Separation: The compounds in the sample are separated as they pass through the stationary and mobile phases.
- Detection: The separated compounds are detected and measured by a detector.

Application in Biology:
- Analysis of proteins and peptides: LC is commonly used to separate and purify proteins and peptides for characterization and identification.
- Analysis of carbohydrates: LC can be used to separate and identify different carbohydrates in a sample.
- Analysis of nucleic acids: LC can be used to separate and purify nucleic acids for downstream applications such as sequencing and cloning.
- Analysis of small molecules: LC can be used to separate and identify small molecules such as drugs and metabolites.
- Other applications of LC include environmental monitoring, food analysis, forensic analysis and pharmaceutical analysis.