Table of Contents
Introduction to Northern blotting:
Northern blotting is a technique used to analyze and identify specific RNA sequences in a sample. The method is based on the principle of RNA separation by electrophoresis and RNA detection by hybridization. It has been widely used in molecular biology, genetics, and medical research for the detection and characterization of RNA.
Discovery of Northern blotting:
This technique was first proposed by the British molecular biologist James Alwine in 1977. He used the method to analyze RNA samples and named it Northern blotting as a reference to the Southern blotting method used to analyze DNA.
Principal of Northern blotting:
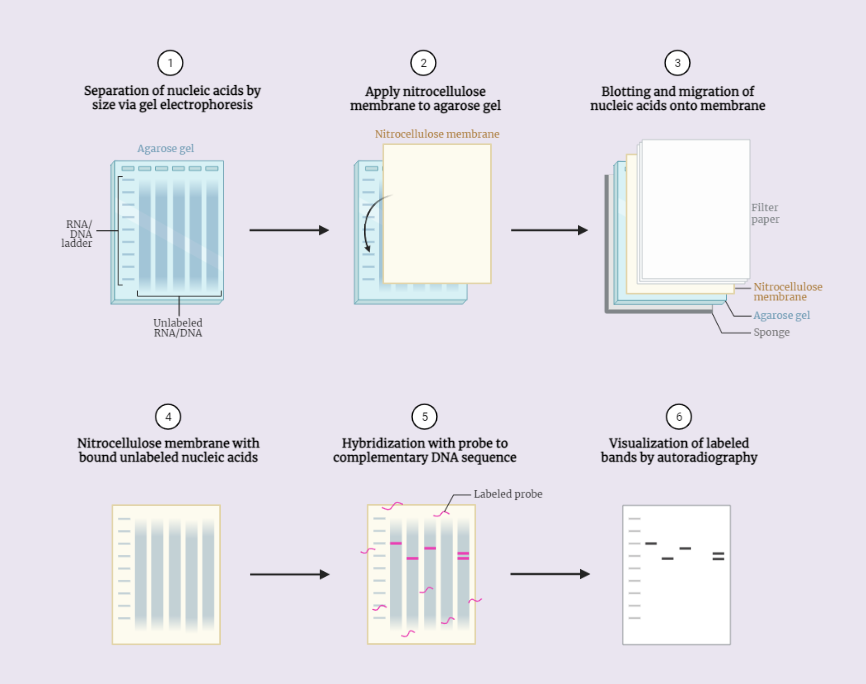
The principle of Northern blotting is based on the separation of RNA by electrophoresis and detection by hybridization. RNA is separated by size and charge in an electrophoresis gel, and then transferred to a membrane, such as nitrocellulose or nylon. The membrane is then incubated with a specific RNA probe, which is a single-stranded DNA or RNA molecule that is complementary to the sequence of interest. The probe binds to the RNA of interest through base-pairing, and then a detection method is used to visualize the hybridized RNA.
Procedure (Step-wise):
- Sample preparation: The RNA sample is prepared by extracting it from the source tissue or cell. The sample is then treated with a reagent such as formaldehyde or ethanol to crosslink the RNA to the gel.
- Electrophoresis: The RNA sample is loaded onto an electrophoresis gel, such as agarose gel, and an electric field is applied. The RNA fragments migrate through the gel based on their size and charge, resulting in a separation of the RNA.
- Transfer: The RNA fragments are transferred from the gel to a membrane, such as nitrocellulose or nylon. This process is called electroblotting or Northern blotting.
- Denaturation: The RNA fragments on the membrane are denatured by heating or chemical treatment to separate the double-stranded RNA into single strands.
- Blocking: The membrane is blocked with a solution containing a non-specific RNA to prevent non-specific binding of the probe.
- Hybridization: The membrane is incubated with a specific RNA probe, which is a single-stranded DNA or RNA molecule that is complementary to the sequence of interest. The probe binds to the RNA of interest through base-pairing.
- Detection: The hybridized RNA is detected by a suitable method such as radioactive or non-radioactive labeling.
- Analysis: The band on the membrane that corresponds to the RNA of interest is analyzed and the RNA is identified.
Applications:
- Medical research: Northern blotting is used to detect and analyze RNA in various diseases such as cancer, genetic disorders, and infectious diseases.
- Genetic engineering: This technique is used to analyze and purify RNA for genetic engineering and gene therapy.
- Quality control: It is used to ensure the quality of RNA samples used in research and industry.
- Diagnostics: This procedure is used in the diagnosis of various diseases such as cystic fibrosis and Huntington’s disease.
- Environmental monitoring: It is used to detect and analyze RNA in environmental samples such as water and soil.
In conclusion, Northern blotting is a powerful technique that is widely used in molecular biology, genetics, and medical research. The method is based on the separation of RNA by electrophoresis and detection by hybridization. Northern blotting has many applications in various fields such as medical research, genetic engineering,