Table of Contents
Introduction
Osmosis is a fundamental process that allows water to move across biological membranes. Understanding the mechanisms of it is essential for grasping various biological processes, including water regulation in living organisms, as well as industrial processes like water purification. In this article, we will explore the basics of osmosis, the factors that affect it, and its significance in various fields.
1. Definition
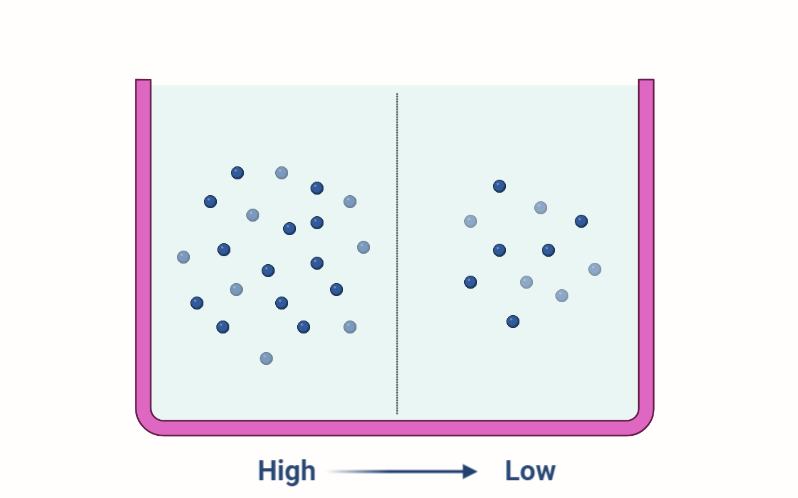
Osmosis is the process by which water moves from an area of high concentration to an area of low concentration across a selectively permeable membrane. This movement is driven by a difference in solute concentration on either side of the membrane.
2. Mechanism
Osmosis occurs due to the random movement of water molecules. When a concentration gradient exists across a membrane, water molecules will move from an area of high concentration to an area of low concentration until equilibrium is reached.

3. Factors Affecting
Several factors can affect the rate and direction of osmosis, including:
- Concentration gradient
- Temperature
- Pressure
- Surface area of the membrane
- Thickness of the membrane
4. Osmotic Pressure
Osmotic pressure is the pressure required to prevent osmosis from occurring. It is dependent on the concentration of solutes on either side of the membrane and can be used to determine the direction of water flow.
5. Osmosis in Living Organisms
Osmosis is a crucial process in the survival of living organisms. It is responsible for the regulation of water balance and the maintenance of cell shape and size.
In plants, it is responsible for the uptake of water and the maintenance of turgor pressure, which allows the plant to remain upright.
In animals, it is responsible for the regulation of fluid balance in the body. It is crucial for the proper functioning of organs such as the kidneys.
6. Importance in Biological Systems
Osmosis plays a crucial role in various biological systems, including water regulation, nutrient uptake, and waste removal. Its significance can be seen in processes such as cell division, digestion, and respiration.
7. Osmosis in Industrial Processes
It has numerous applications in industrial processes, including water purification and desalination. It is used in reverse osmosis systems to remove impurities and produce clean water.
8. Reverse Osmosis
Reverse osmosis is a process that uses pressure to force water through a semipermeable membrane, removing impurities and producing clean water.
9. Applications
Osmosis has numerous applications in various fields, including medicine, agriculture, and industry. It is used in drug delivery systems, food preservation, and wastewater treatment, among other things.
10. Conclusion
In conclusion, osmosis is a vital biological process that is essential for life. It has significant implications for human health and plays a crucial role in biological processes such as nutrient and waste transport and water management.