Table of Contents
Introduction:
Real-Time PCR is a powerful and versatile tool used in molecular biology to quantify the amount of specific DNA or RNA in a sample. This technique enables quantification of nucleic acids in real-time by monitoring the accumulation of fluorescence generated during the extension phase of the PCR reaction.
Principle:
Real-Time PCR is based on the polymerase chain reaction (PCR), which is a laboratory technique used to amplify specific regions of DNA. The basic principle of Real-Time PCR is to monitor the accumulation of fluorescence produced by the reaction during each cycle, allowing for real-time quantification of the target DNA.
Reagents:
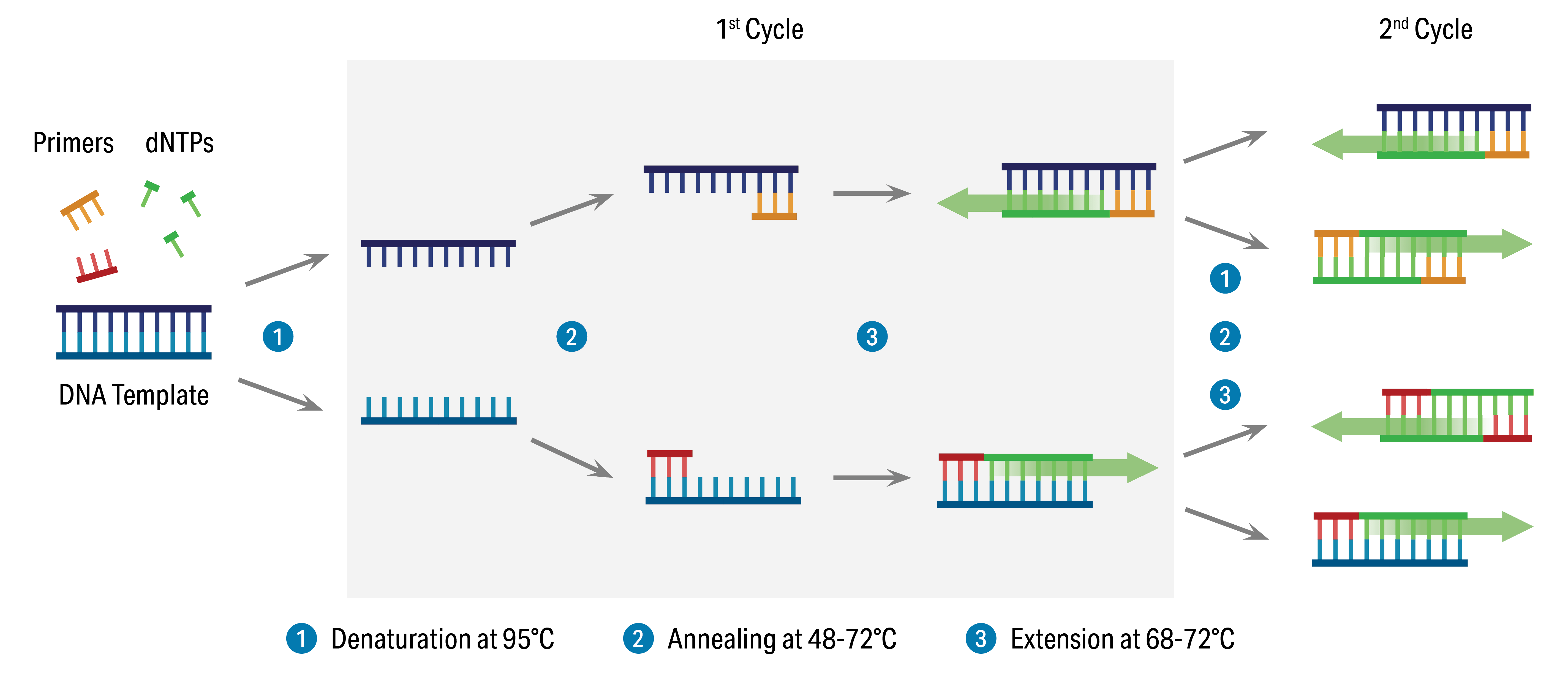
The components required for Real-Time PCR include the template DNA, primers, Taq polymerase, dNTPs, buffer, and fluorescent dye or probe. The template DNA is the target DNA that needs to be quantified. Primers are short DNA sequences that define the region of the template DNA that will be amplified. Taq polymerase is an enzyme that synthesizes new DNA strands. dNTPs are the building blocks of DNA. Buffer contains the necessary salts and enzymes required for the PCR reaction. Fluorescent dyes or probes can be used to monitor the reaction and quantify the target DNA.
Steps in the Real-Time PCR Process:
- Template DNA extraction and purification: The first step in the Real-Time PCR process is the extraction and purification of the template DNA from the sample. This can be done using various methods such as spin-column, silica-membrane, or bead-based purification.
- Primer design: The next step is to design the primers that will be used to define the region of the template DNA that will be amplified. Primers should be specific to the target DNA and have the appropriate melting temperature (Tm) and length.
- Master mix preparation: A master mix is prepared by mixing all the reagents required for the PCR reaction in a single tube. This includes the template DNA, primers, Taq polymerase, dNTPs, buffer, and fluorescent dye or probe.
- Thermal cycling: The master mix is then subjected to thermal cycling, which involves repeated heating and cooling cycles to initiate and amplify the PCR reaction. During each cycle, the reaction mixture is subjected to different temperatures to activate the Taq polymerase, denature the double-stranded DNA, and allow the primers to anneal to the template DNA.
- Real-time quantification: During the thermal cycling, the fluorescent dye or probe generates fluorescence that can be monitored in real-time. The fluorescence is proportional to the amount of target DNA in the sample.
- Data analysis: After the thermal cycling is complete, the data generated by the real-time monitoring is analyzed using specialized software. This software can calculate the amount of target DNA present in the sample, compare the results with a reference sample, or determine the relative expression of genes in the sample.
Applications:
Real-Time PCR has a wide range of applications in molecular biology, including the quantification of DNA and RNA, the detection of pathogenic microorganisms, and the analysis of gene expression. It is widely used in medical and scientific research, forensics, and diagnostics.
Advantages:
- High sensitivity and specificity: Real-Time PCR is highly sensitive and specific, allowing for the quantification of low amounts of target DNA or RNA.
- Fast and efficient: The real-time monitoring of the PCR reaction enables faster and more efficient quantification
- of target DNA compared to traditional PCR methods.
- Multiplexing: Real-Time PCR can be multiplexed, allowing for the simultaneous detection and quantification of multiple targets in a single reaction.
- Automation: Real-Time PCR can be automated, enabling high-throughput analysis of large numbers of samples.
- Cost-effective: The cost-effectiveness of Real-Time PCR is increasing with advancements in technology and reagents, making it accessible for many laboratory and diagnostic applications.
Limitations:
- Interferences: Interferences from inhibitors in the sample can affect the accuracy of the Real-Time PCR results.
- Variations in thermal cyclers: Variations in thermal cyclers can lead to differences in results, making it important to standardize the conditions for the PCR reaction.
- Non-specific binding: Non-specific binding of the primers or probes to regions of the template DNA that are not the target can result in false positive results.
Conclusion:
Real-Time PCR is a powerful and widely used tool in molecular biology, providing fast, efficient, and sensitive quantification of target DNA or RNA. With continued advancements in technology and reagents, Real-Time PCR will continue to play a critical role in many scientific and medical applications.